If these things to do are completed consistently, it noticeably increases the item quality and lowers item failure.
Our auditors have won the continuing rely on of a lot more than 1600 consumers and may help you to determine and mitigate the intrinsic hazard within your functions, provide chains and procedures.
A GMP audit is a comprehensive, 3rd-party inspection of pharmaceutical manufacturing company or supplier in the pharmaceutical worth chain.
Pharmaceutical companies use GMP audits to validate producing controls and permit timely issue correction. Management audits comprehensively take a look at an organization. Audits may be very first, 2nd, or third party. An auditor's obligations contain offering audit studies and determining challenges. The scheduling process for an audit entails asserting a agenda, conducting meetings, undertaking the audit, and delivering abide by-up.
The auditor shall be in search of evidence of your company’s compliance having a system of action produced inside the reaction into the warning letter.
For The entire supply chain, this Shared Audit strategy drives favourable environmental impression likewise. Combining numerous specific audits into a single cuts down the footprint from vacation.
Audit trail evaluation is a responsible do the job and it could possibly be certain by the other department as an alternative to consumer Section. The regulatory expects that there really should not be any bias assessment around the audit trail.
With SimplerQMS, you can easily obtain paperwork and reports through the merchandise lifestyle cycle, in an individual place. This makes it easy to share information with external associates.
This document discusses different types of high quality audits, including internal audits conducted by a company to be sure top quality expectations are achieved, exterior audits executed by outside get-togethers to make sure benchmarks are achieved for suppliers or consumers, and various focused audits like method, merchandise, and system audits.
QUALIFICATION & VALIDATION.Validation is A vital Section of GMP, and a component of QA.Vital actions in the process must be validated.Want for assurance that the solution will continuously meet predetermined specs and characteristics.
External audits seek advice from audits executed by shoppers on suppliers or contractors. Regulatory click here audits are carried out by independent regulatory bodies much like the FDA to make sure compliance with suitable rules. The audits assistance companies Consider processes, determine difficulties, and guarantee top quality benchmarks are met.
Danger Mitigation: Audits support establish prospective hazards and vulnerabilities in procedures, provide chain, and documentation, enabling companies to implement actions to mitigate Those people pitfalls correctly.
To elucidate the company policy on any click here denial, exactly where the company just isn't ready to present the information to your Auditor.
Importance of Audit Scheduling: Talk about the significance of complete audit planning to make certain audits are very well-structured and centered on crucial places.
 Alicia Silverstone Then & Now!
Alicia Silverstone Then & Now! Devin Ratray Then & Now!
Devin Ratray Then & Now!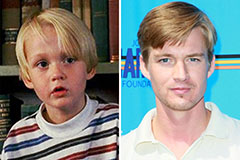 Mason Gamble Then & Now!
Mason Gamble Then & Now!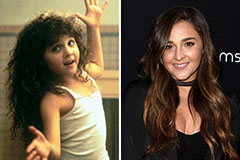 Alisan Porter Then & Now!
Alisan Porter Then & Now! Teri Hatcher Then & Now!
Teri Hatcher Then & Now!